In this water exercise, we focused on the manipulation of water and how the properties of water changed as we worked with it. We used resources like clay, salt, sand and various sponges to see how the properties of water varied as we changed our scenarios. Some properties like cohesion, adhesion, and solvent properties emerged in our observations. In our past lectures, we discussed the properties of water including surface tension, Turgor pressure, cohesion and adhesion. Similarly, we also observed scenarios of water in nature and found that some of the properties seen in nature were also seen in our own experimentation.

The water molecules stick to all of the materials in little droplets. The molecules stick to all objects except for the sponge which absorbs the water. Using something different this time, the metal ring, we observed surface tension again as the molecules stuck together. When using the dish washing sponge we saw that cohesion was present. When water hit the sponge, cohesion began and with time, surface tension broke and water flowed through the object, no longer one unified mass. Once the water drained through the pad of the blue sponge, surface tension and cohesion were present in the green tub of water. The rope seemed to only absorb the water when left in the water for a long time. The texture changed from coarse to slightly soft with the addition of water to the rope. Similarly, the texture of the orange sponge changed as well; as it expanded in size it became plush in texture with the addition of water. With clay, water adheres to the material but cohesion is not present as the water molecules do not become one unified whole on the clay material. We have concluded that the water does not change the composition of the material, rather it changes the texture and how the material sometimes feels. The water doesn’t change other than the new presence of cohesion, adhesion, absorption or surface tension. The materials change in texture and sometimes in size rather, as the water acts as one unified cohesive unit with the addition of materials, unless separated by the absorption of molecules.
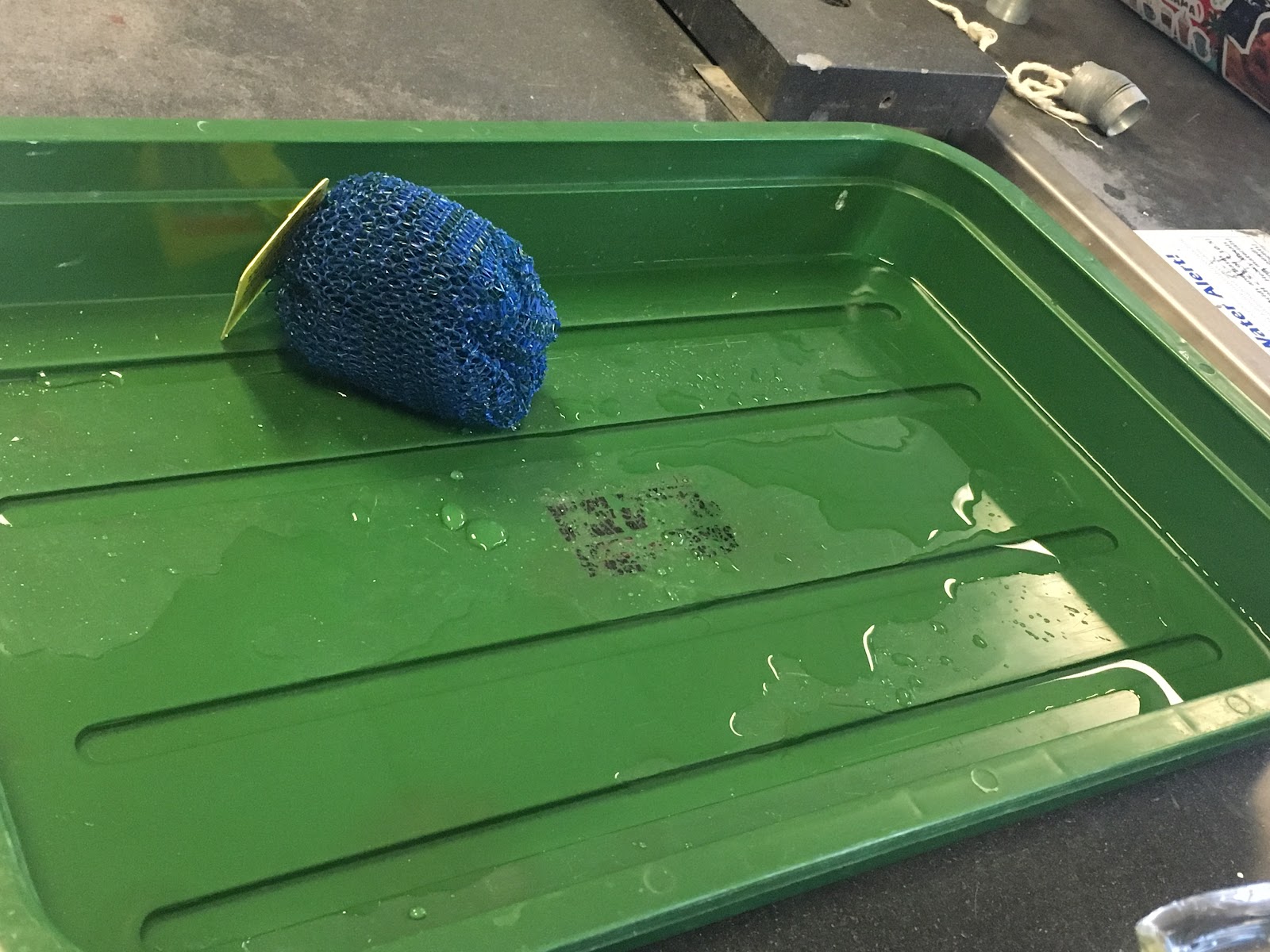
To manipulate the flow of water, we held a dishwashing pad over a green tub, and poured water through the pad. The first thing we noticed was the change in the flow of water: instead of flowing as one stream, the water conformed to the shape of the pad, some of the water flowed off of the side of the pad, and began to flow through the tiny holes in the pad. However, the material didn’t absorb the water, it just changed the way the water flowed together, while maintaining the same texture, only becoming wet. The most evident properties of water in this experiment were adhesion and cohesion. For instance, after the water flowed through the pad, one still saw remnants of water, through the few water droplets left on the pad, this is due to adhesion. However, the reason that the water formed droplets instead of laying flat is due to Turgor pressure, where water molecules push so strongly against each other, that they are able to maintain a rounded structure. Once the flow of water hit the green tub, you are able to see cohesive properties. Instead of flowing all over the tub, the water stuck together, creating mass flow (the phenomenon of water flowing together as a single mass) and a puddle with surface tension. To test this cohesion, we placed the pad in the puddle. While this did disrupt some of the cohesion, for the most part, the pad was unable to fully disrupt the surface tension, and upon removal of the pad, the water remained in the exact same puddle. This shows that the cohesive properties of the water may be stronger than the adhesive properties, in regards to the dishwashing pad.
This lab helped make something ‘invisible’ into something visible through the obvious presence of adhesion and cohesion. Adhesion allows us to see water molecules stick to other things, for example, a dishwashing pad. The water molecules separated when we poured the water onto the dishwashing pad as they flowed through the pad. Some droplets stuck to the pad as the rest flowed through. They then flowed onto the green pan where they gathered in a ‘puddle’ and formed as surface tension. The presence of surface tension is a visible example of the invisible hydrogen bonds coming together through cohesion. Through close observation of this behavior, and learning in lecture the cause of this behavior and how it subsequently presence itself, we were able to make minute concepts seem more present.
This lab made the properties of water that contribute to its motion and behavior much more visible. When we pour water into a glass, we don’t think about what’s happening to the actual water beyond “It is going into a glass and when it is done, I will drink it.” When we take time to specifically observe the activity, it suddenly becomes “the water is cohering to itself, adhering to the sides of the glass, and exhibiting mass flow. The necessity of observation takes an ordinary experience and turn it into a demonstration of scientific concept and exploration.
Additionally, this lab provided the visual and tactile, sensory engagement necessary to make large concepts more real. While surface tension is simple enough to explain in lecture, it’s much more beneficial to see it in action. When one sees a water droplet clinging to itself and its surface, the properties of cohesion, adhesion and surface tension suddenly become much more real.
Visibility depends on size because the scale of a process because if something is too big or too microscopic, it can be more difficult to understand. We don’t know exactly what single water molecules flowing up a xylem tube looks like, and sometimes it’s difficult to imagine exactly how water flows in mass quantities if you haven’t spent a lot of time near large bodies of water. Today, we were able to create our own scenarios of how water should move, and it was easier to see and understand because it was in a relatively normal scale. We were able to see mass flow in a controlled environment, which made it easier to visualize on the other ends of the scale spectrum.